T Cells: The Body’s Front Line Cancer Fighters — And How to Support Them Naturally

How T‑cells detect and destroy cancer cells, how immunotherapy strengthens them, and what lifestyle, nutrition, supplements, and repurposed medications may support healthy T‑cell function.
Researched and written by Keith Bishop, Integrative Cancer Educator, Cancer Coach, Clinical Nutritionist, Retired Pharmacist, and Founder of Prevail Over Cancer.
Why T‑Cells Matter More Than Ever in Cancer Defense
Over the last decade, T‑cells have become the centerpiece of modern cancer treatment. Immunotherapies such as checkpoint inhibitors, CAR T therapy, and cancer vaccines all work by activating or enhancing T cell function. This is because T‑cells are the immune system’s precision-guided assassins, capable of identifying and destroying cancer cells with extraordinary specificity.
A central review on T cells and cancer immunology emphasizes that T cells are the primary drivers of antitumor immunity, orchestrating both direct killing and broader immune coordination.[i] Without effective T‑cell activity, cancer can grow unchecked. When T cells are activated, expanded, or re-energized, tumors can shrink — sometimes dramatically.
Recent breakthroughs have revealed even more about how T‑cells work. Scientists have discovered that the T cell receptor (TCR) activates through a hidden “spring-loaded” mechanical switch that snaps open when it encounters a suspicious antigen.[ii] This discovery helps explain why T‑cells are so effective at detecting cancer cells — and why immunotherapy works for some patients but not others.
Understanding how T‑cells function — and how to support them — is essential for anyone navigating cancer, prevention, or immune health.
Check out the FREE 20-minute Empowered Cancer Workshop.
There's one starting in a few minutes.
How T‑Cells Detect and Destroy Cancer Cells
- T‑Cell Recognition: The TCR as a Precision Sensor
T‑cells constantly patrol the body, scanning for abnormal peptides displayed on cell surfaces. The TCR is the molecular “eye” that recognizes these antigens.
Recent structural research shows that the TCR behaves like a jack-in-the-box, remaining compact until it encounters an antigen, then rapidly opening to initiate activation.[iii]
This mechanical activation is foundational to all T‑cell–based cancer therapies.
- Cytotoxic Killing: How T‑Cells Destroy Tumor Cells
Once activated, CD8+ cytotoxic T‑cells directly kill cancer cells using:
- Perforin — creates pores in the cancer cell membrane[iv]
- Granzymes — enter through pores and trigger apoptosis[v]
- Fas–FasL signaling — induces programmed cell death[vi]
These mechanisms are the same ones leveraged in CAR‑T therapy and checkpoint inhibitors.
- Immune Coordination: T‑Cells Amplify the Anti-Cancer Response
Activated T‑cells release cytokines such as interferon‑gamma (IFN‑γ) that:
- Increase antigen presentation
- Recruit additional immune cells
- Slow tumor growth
- Enhance immune visibility of cancer cells
This makes T‑cells the architects of systemic antitumor immunity.[vii]
- T‑Cell Exhaustion: A Major Barrier in Cancer
Tumors often create an immunosuppressive environment that leads to T-cell exhaustion — a dysfunctional state in which T-cells lose their ability to kill cancer cells effectively.
Checkpoint inhibitors (e.g., anti-PD1, anti-CTLA4) work by releasing the brakes on exhausted T‑cells, restoring their ability to attack tumors.[viii]
How Modern Cancer Treatments Enhance T‑Cell Function
Nearly every major immunotherapy works by activating, expanding, or re-energizing T cells.
Checkpoint Inhibitors
These drugs block inhibitory pathways (PD-1, CTLA-4) that tumors use to disable T‑cells.
By removing these brakes, T‑cells regain their ability to kill cancer cells.
CAR‑T Therapy
CAR T therapy genetically engineers a patient’s T cells to better recognize cancer cells.
This approach is built entirely on T‑cell cytotoxicity.
Cancer Vaccines
Cancer vaccines train T‑cells to recognize tumor antigens more effectively.
Emerging Approaches
The newly discovered spring-loaded TCR mechanism may lead to next-generation immunotherapies that activate T‑cells more efficiently.
Lifestyle Strategies That Support Healthy T‑Cell Activity
While lifestyle cannot replace medical treatment, certain habits support a healthier immune environment.
- Exercise
Moderate physical activity enhances immune surveillance and T‑cell circulation.[ix]
- Stress Reduction
Chronic stress elevates cortisol, which suppresses T‑cell activity.[x]
- Sleep
Sleep is essential for immune regulation and T‑cell signaling.[xi]
- Avoiding Smoking & Excess Alcohol
Both impair immune cell function and increase oxidative stress.[xii] [xiii]
The T‑Cell Secret Behind Fasting
The T Cell Secret Behind Fasting Many people think intermittent fasting is just about ketones - but the real magic happens deep in your immune system. Emerging research shows that short-term fasting can recharge T cells, reduce immune exhaustion, and improve the quality of your anticancer immune response. T cells are your body’s primary defense against cancer.
I’ve got five benefits of fasting and T-Cells next. But if you want the full story, comment or message TCELL for a link to our updated T-Cell Blog.
Here’s what the research shows:
- Fasting activates autophagy in immune cells, helping T cells clear damaged proteins and improve function
- Short-term fasting enhances T cell stress resistance and improves memory T cell formation
- Fasting reduces systemic inflammation, lowering the metabolic stress that drives T cell exhaustion
- Intermittent fasting improves metabolic flexibility, supporting mitochondrial efficiency — a key requirement for high-quality T cell activation
- Fasting boosts dendritic cell activity, improving antigen presentation and T cell priming
Together, these shifts create a more resilient, less exhausted, more metabolically capable T cell population — exactly what you want when supporting immune surveillance during cancer challenges.
Foods That Support T‑Cell Health
Selenium‑Rich Foods: Brazil Nuts & Sunflower Seeds
Selenium supports T‑cell proliferation, antioxidant defense, and immune signaling.
Research shows that selenium-dependent selenoproteins are essential for T cell expansion and survival.[xiv]
Garlic and T-cells
Garlic’s sulfur compounds stimulate lymphocytes that support immune surveillance and anticancer activity.[xv]
Green Tea EGCG and T-cells
EGCG supports immune modulation and antioxidant protection.[xvi]
Omega-3–Rich Fish and T-cells
Supports balanced inflammatory signaling and immune regulation.[xvii]
Fermented Foods and T-cells
Probiotics influence regulatory T‑cell pathways through gut‑immune communication.[xviii]
Supplements With Evidence for Supporting T‑Cell Anti-cancer Function
(General educational information — not medical advice.)
- Vitamin D — supports T‑cell activation[xix]
- Zinc — essential for T‑cell development[xx]
- Selenium — supports proliferation and immune signaling[xxi]
- Vitamin A — supports T‑cell differentiation[xxii]
- Green tea extract (EGCG) — immune‑modulating polyphenols[xxiii]
- Probiotics — influence regulatory T‑cell pathways[xxiv]
- Berberine – helps T-cell infiltration into cancer cells[xxv]
- Curcumin – Restores T-cell activity[xxvi]
- Quercetin – improves T-cell anti-tumor responses[xxvii]
- Boswellia / Frankincense – improves T-cell priming and cytotoxicity[xxviii]
- Resveratrol – activates tumor-infiltrating CD8* T-cells[xxix]
- Fisetin – induces tumor apoptosis (cell death) and increases T-cell priming[xxx]
- Beta-glucan – activates macrophages and supports T-cell responses[xxxi]
- D-mannose – enhances mitochondrial (energy) function in T-cells[xxxii]
- Melatonin – binds to receptors on T-cells and influences their activation and differentiation towards a more anti-tumor phenotype[xxxiii]
- Ashwagandha – increases T-cell levels and lymphocyte function[xxxiv]

Onco-Adjunct Pathway Supplements are a core part of my Prevail Protocol.
Repurposed Medications Being Studied for T-cell Cancer Immune Support
(Educational only — not standard cancer treatment.)
- Metformin — influences immune metabolism[xxxv]
- Statins — anti-inflammatory and immune-modulating properties, potentiate CD8 T-cell activity and efficacy of anti-PD-1 (checkpoint inhibition) immune therapy[xxxvi]
- Mebendazole – increases ERK activity in CD4* T-cells[xxxvii]
- Cimetidine – suppresses suppressor T lymphocyte activity improving cell-mediated anti-tumor immunity[xxxviii]
- Loratadine – higher doses cause apoptosis/pyroptosis, increasing tumor antigens and priming T-cells and checkpoint inhibitor response[xxxix] [xl]
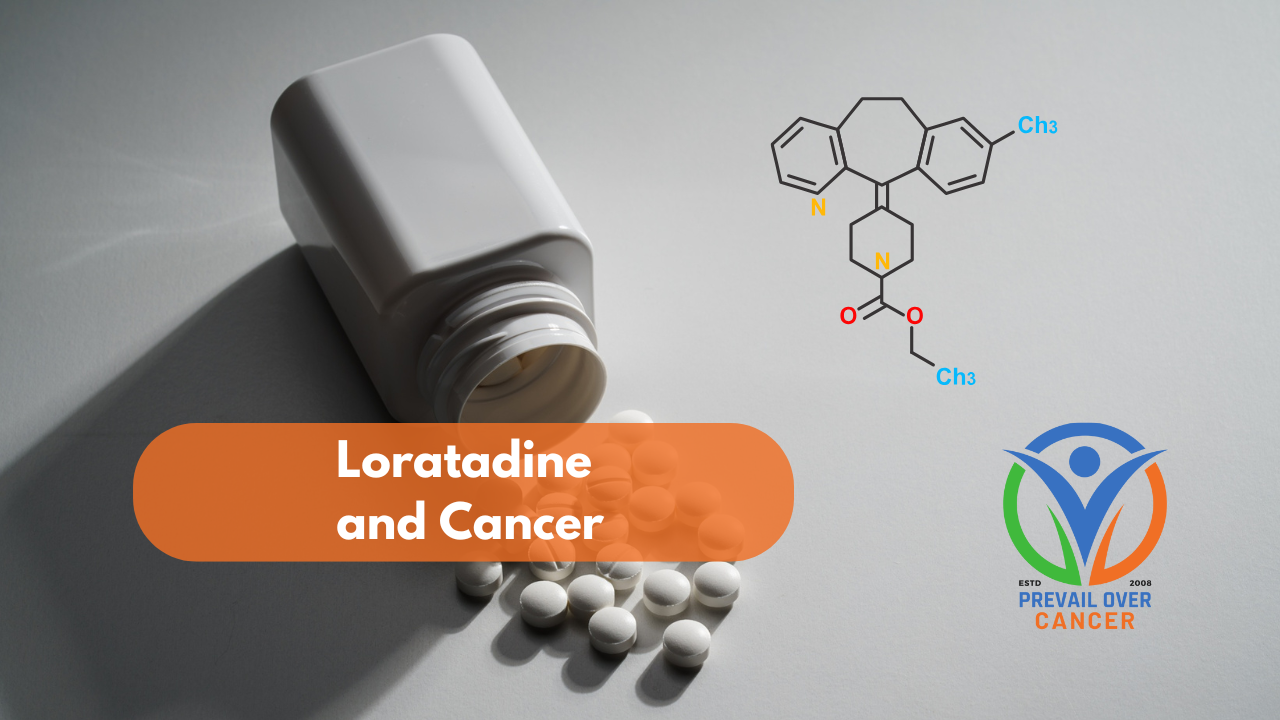
Tap or click here to download the FREE Loratadine and Cancer Clinician Guide - Propranolol - supports T-cell anti-tumor immunity, particularly in combination with checkpoint inhibitors[xli]
- Mistletoe – causes cancer cell death and primes T-cell responses[xlii]
These are areas of active research and not standard-of-care cancer treatments.
Conclusion: Why Supporting T‑Cells for Cancer Matters
T‑cells are the core machinery behind the body’s ability to detect and destroy cancer cells. They are also the target of nearly every major immunotherapy breakthrough of the last decade.
Supporting T‑cell health through:
- medical treatment
- lifestyle
- nutrition
- supplements
- and emerging repurposed medications
…helps strengthen the overall immune terrain.
Understanding how T cells work empowers people with cancer, caregivers, and healthcare clinicians to make informed, evidence-based decisions that support the body’s natural anticancer defenses.
T-cell Cancer Reference Sources
[i] Chen, C., Liu, X., Chang, Y., Wang, H. Y., & Wang, F. (2023). The Interplay between T Cells and Cancer: The Basis of Immunotherapy. Genes, 14(5), 1008. https://doi.org/10.3390/genes14051008
[ii] Notti, R. Q., Yi, F., Heissel, S., Bush, M. W., Molvi, Z., Das, P., Molina, H., Klebanoff, C. A., & Walz, T. (2025). The resting and ligand-bound states of the membrane-embedded human T-cell receptor–CD3 complex. Nature Communications, 16(1), 10996. https://doi.org/10.1038/s41467-025-66939-7
[iii] Sanz-Garcia E, Soleimani S, Bruce JP, et al. Investigating Early Kinetics in Plasma ctDNA and Peripheral T Cell Receptor Repertoire to Predict Treatment Outcomes to PD-1 Inhibitors in Head and Neck Cancer. Clin Cancer Res. Published online December 19, 2025. doi:10.1158/1078-0432.CCR-25-3541 https://pubmed.ncbi.nlm.nih.gov/41417470/
[iv] Friedmann D, Payne KJ, Cousin V, et al. Expansion of a distinct cytotoxic CD4 TFH-cell cluster in lymph nodes of patients with complicated common variable immunodeficiency. Journal of Allergy and Clinical Immunology. Published online November 1, 2025. doi: https://doi.org/10.1016/j.jaci.2025.09.032
[v] Arjumand S, Raj A, Prattay KMR, Omer HBM, Azam F. Chimeric antigen receptor T cell therapy: Revolutionizing cancer treatment. World J Clin Oncol. 2025;16(11):108667. doi:10.5306/wjco.v16.i11.108667 https://pubmed.ncbi.nlm.nih.gov/41355907/
[vi] Wu, ZX., Da, TT., Huang, C. et al. CD69+CD103+CD8+ tissue-resident memory T cells possess stronger anti-tumor activity and predict better prognosis in colorectal cancer. Cell Commun Signal 22, 608 (2024). https://doi.org/10.1186/s12964-024-01990-3
[vii] Brown JR, Sonpavde G. Toward a better pan-tumor predictive signature for unleashing precision immuno-oncology. Journal for ImmunoTherapy of Cancer. 2025;13(12):e013213. doi: https://doi.org/10.1136/jitc-2025-013213
[viii] Xia Y, Zhu J, Guo R, et al. Preclinical evaluation of TIGIT as a target to enhance efficacy and mitigate T cell exhaustion in multiple myeloma following BCMA-CAR-T therapy. Cell Death Dis. 2025;16(1):890. Published 2025 Dec 19. doi:10.1038/s41419-025-08203-w https://pubmed.ncbi.nlm.nih.gov/41419632/
[ix] He A, Bao T, Rong S, et al. Exercise-Induced Metabolic Reprogramming and Immune Modulation: A Novel Strategy for Cancer Therapy. Exerc Immunol Rev. 2025;31:19-35. https://pubmed.ncbi.nlm.nih.gov/41408794/
[x] Globig, A., Zhao, S., Roginsky, J., Maltez, V. I., Guiza, J., Heeg, M., Araujo Hoffmann, F., Chaudhary, O., Wang, J., Senturk, G., Chen, D., Pfaff, S., Germain, R. N., Schalper, K. A., Emu, B., & Kaech, S. M. (2023). The β1-adrenergic receptor links sympathetic nerves to T cell exhaustion. Nature, 622(7982), 383-392. https://doi.org/10.1038/s41586-023-06568-6
[xi] Singh KK, Ghosh S, Bhola A, et al. Sleep and Immune System Crosstalk: Implications for Inflammatory Homeostasis and Disease Pathogenesis. Annals of Neurosciences. Published online September 20, 2024. doi: https://doi.org/10.1177/09727531241275347
[xii] Bundell, S., & Petrić Howe, N. (2024). Smoking changes your immune system, even years after quitting. https://doi.org/10.1038/d41586-024-00482-1
[xiii] McTernan, P. M., Levitt, D. E., Welsh, D. A., Simon, L., Siggins, R. W., & Molina, P. E. (2022). Alcohol Impairs Immunometabolism and Promotes Naïve T Cell Differentiation to Pro-Inflammatory Th1 CD4+ T Cells. Frontiers in Immunology, 13, 839390. https://doi.org/10.3389/fimmu.2022.839390
[xiv] Ren, F., Chen, X., Hesketh, J., Gan, F., & Huang, K. (2012). Selenium Promotes T-Cell Response to TCR-Stimulation and ConA, but Not PHA in Primary Porcine Splenocytes. PLOS ONE, 7(4), e35375. https://doi.org/10.1371/journal.pone.0035375
[xv] Arreola, R., Quintero-Fabián, S., López-Roa, R. I., Flores-Gutiérrez, E. O., Reyes-Grajeda, J. P., Carrera-Quintanar, L., & Ortuño-Sahagún, D. (2015). Immunomodulation and Anti-Inflammatory Effects of Garlic Compounds. Journal of Immunology Research, 2015(1), 401630. https://doi.org/10.1155/2015/401630
[xvi] Pae M, Wu D. Immunomodulating effects of epigallocatechin-3-gallate from green tea: mechanisms and applications. Food & Function. 2013;4(9):1287. doi: https://doi.org/10.1039/c3fo60076a
[xvii] Shaikh, S. R. (2020). Omega-3s are a traffic light for T cells: Lipid metabolites and membrane-related events at the crossroads of inflammation. Cardiovascular Research, 116(5), 874-875. https://doi.org/10.1093/cvr/cvz220
[xviii] New study reveals how kimchi boosts the immune system. ScienceDaily. Published 2025. https://www.sciencedaily.com/releases/2025/12/251216081945.htm
[xix] Fisher, S. A., Rahimzadeh, M., Brierley, C., Gration, B., Doree, C., Kimber, C. E., Cajide, A. P., Lamikanra, A. A., & Roberts, D. J. (2019). The role of vitamin D in increasing circulating T regulatory cell numbers and modulating T regulatory cell phenotypes in patients with inflammatory disease or in healthy volunteers: A systematic review. PLOS ONE, 14(9), e0222313. https://doi.org/10.1371/journal.pone.0222313
[xx] Perkey E, Maillard I. Zinc: a damage signal promoting thymic repair. Blood. 2022;139(25):3569-3570. doi: https://doi.org/10.1182/blood.2022016333
[xxi] Ren, F., Chen, X., Hesketh, J., Gan, F., & Huang, K. (2012). Selenium Promotes T-Cell Response to TCR-Stimulation and ConA, but Not PHA in Primary Porcine Splenocytes. PLOS ONE, 7(4), e35375. https://doi.org/10.1371/journal.pone.0035375
[xxii] Hall Jason A, Cannons Jennifer L, Grainger John R, et al. Essential Role for Retinoic Acid in the Promotion of CD4+ T Cell Effector Responses via Retinoic Acid Receptor Alpha. Immunity. 2011;34(3):435-447. doi: https://doi.org/10.1016/j.immuni.2011.03.003
[xxiii] Talib, W. H., Awajan, D., Alqudah, A., Alsawwaf, R., Althunibat, R., Abu AlRoos, M., Al Safadi, A., Abu Asab, S., Hadi, R. W., & Al Kury, L. T. (2024). Targeting Cancer Hallmarks with Epigallocatechin Gallate (EGCG): Mechanistic Basis and Therapeutic Targets. Molecules, 29(6), 1373. https://doi.org/10.3390/molecules29061373
[xxiv] Liu, Y., Cao, X., Liu, H., & Zhang, W. (2025). The crosstalk between probiotics and T cell immunity. Frontiers in Immunology, 16, 1695840. https://doi.org/10.3389/fimmu.2025.1695840
[xxv] Zhang, X., Xiong, B., Cheng, Y., Huang, J., Xue, J., Li, X., Lu, W., Zhu, J., Wang, L., Yang, W., & Cheng, Z. (2025). Berberine inhibits metastasis of ovarian cancer by blocking lipid metabolism, alleviating aging of adipose tissue and increasing tumor infiltrating immune cells. Translational Oncology, 56, 102380. https://doi.org/10.1016/j.tranon.2025.102380
[xxvi] Bhattacharyya, S., Mohanty, S., Sankar Sen, G., Chattopadhyay, S., Banerjee, S., Chakraborty, J., Das, K., Sarkar, D., Das, T., & Sa, G. (2010). Curcumin reverses T cell-mediated adaptive immune dysfunctions in tumor-bearing hosts. Cellular & Molecular Immunology, 7(4), 306-315. https://doi.org/10.1038/cmi.2010.11
[xxvii] Han P, Chu S, Shen J, et al. Quercetin-derived microbial metabolite DOPAC potentiates CD8+ T cell anti-tumor immunity via NRF2-mediated mitophagy. Cell Metabolism. 2025;37(12):2438-2454.e8. doi: https://doi.org/10.1016/j.cmet.2025.09.010
[xxviii] Trivedi, V. L., Soni, R., Dhyani, P., Sati, P., Tejada, S., Sureda, A., Setzer, W. N., Modu, B., & Butnariu, M. (2023). Anti-cancer properties of boswellic acids: Mechanism of action as anti-cancerous agent. Frontiers in Pharmacology, 14, 1187181. https://doi.org/10.3389/fphar.2023.1187181
[xxix] Zhang, W., Zhang, R., Chang, Z., & Wang, X. (2022). Resveratrol activates CD8+ T cells through IL-18 bystander activation in lung adenocarcinoma. Frontiers in Pharmacology, 13, 1031438. https://doi.org/10.3389/fphar.2022.1031438
[xxx] Fatima, R., Soni, P., Sharma, M. et al. Fisetin as a chemoprotective and chemotherapeutic agent: mechanistic insights and future directions in cancer therapy. Med Oncol 42, 104 (2025). https://doi.org/10.1007/s12032-025-02664-x
[xxxi] Ameri Shah Reza, M., Najafi, S., Kahfi, M. et al. Beta-glucans in oncology: revolutionizing treatment with immune power & tumor targeting. Naunyn-Schmiedeberg's Arch Pharmacol (2025). https://doi.org/10.1007/s00210-025-04519-8
[xxxii] Qiu Y, Su Y, Xie E, et al. Mannose metabolism reshapes T cell differentiation to enhance anti-tumor immunity. Cancer Cell. 2024;43(1):103-121.e8. doi: https://doi.org/10.1016/j.ccell.2024.11.003
[xxxiii] Wang L, Wang C, Choi WS. Use of Melatonin in Cancer Treatment: Where Are We? International Journal of Molecular Sciences. 2022;23(7):3779. doi: https://doi.org/10.3390/ijms23073779
[xxxiv] Tharakan, A., Shukla, H., Benny, I. R., Tharakan, M., George, L., & Koshy, S. (2021). Immunomodulatory Effect of Withania somnifera (Ashwagandha) Extract—A Randomized, Double-Blind, Placebo Controlled Trial with an Open Label Extension on Healthy Participants. Journal of Clinical Medicine, 10(16), 3644. https://doi.org/10.3390/jcm10163644
[xxxv] Finisguerra V, Tereza Dvorakova, Matteo Formenti, et al. Metformin improves cancer immunotherapy by directly rescuing tumor-infiltrating CD8 T lymphocytes from hypoxia-induced immunosuppression. Journal for immunotherapy of cancer. 2023;11(5):e005719-e005719. Doi: https://doi.org/10.1136/jitc-2022-005719
[xxxvi] Correction: Statin drugs enhance responses to immune checkpoint blockade in head and neck cancer models. Journal for ImmunoTherapy of Cancer. 2023;11(2):e005940corr1. doi: https://doi.org/10.1136/jitc-2022-005940corr1
[xxxvii] Andersson CR, Selvin T, Blom K, et al. Mebendazole is unique among tubulin-active drugs in activating the MEK–ERK pathway. Scientific Reports. 2020;10(1). doi: https://doi.org/10.1038/s41598-020-68986-0
[xxxviii] Kubota T, Fujiwara H, Ueda Y, et al. Cimetidine modulates the antigen presenting capacity of dendritic cells from colorectal cancer patients. British Journal of Cancer. 2002;86:1257-1261. doi: https://doi.org/10.1038/sj/bjc/6600233
[xxxix] Liu X, Zhong R, Huang J, et al. Loratidine is associated with improved prognosis and exerts antineoplastic effects via apoptotic and pyroptotic crosstalk in lung cancer. Journal of Experimental & Clinical Cancer Research. 2024;43(1). doi: https://doi.org/10.1186/s13046-023-02914-8
[xl] Release MAN. Antihistamines can influence immunotherapy response by enhancing T cell activation. MD Anderson Cancer Center. https://www.mdanderson.org/newsroom/antihistamines-may-improve-immunotherapy-responses-study-finds.h00-159465579.html
[xli] O’Logbon, J., Tarantola, L., Williams, N.R. et al. Does propranolol have a role in cancer treatment? A systematic review of the epidemiological and clinical trial literature on beta-blockers. J Cancer Res Clin Oncol 151, 212 (2025). https://doi.org/10.1007/s00432-025-06262-2
[xlii] Hong, C., & Lyu, S. (2025). Mistletoe in Cancer Cell Biology: Recent Advances. Current Issues in Molecular Biology, 47(8), 672. https://doi.org/10.3390/cimb47080672
Fasting References
Madeo F, Carmona-Gutierrez D, Hofer SJ, Kroemer G. Caloric Restriction Mimetics against Age-Associated Disease: Targets, Mechanisms, and Therapeutic Potential. Cell Metabolism. 2019;29(3):592-610. doi: https://doi.org/10.1016/j.cmet.2019.01.018
Longo Valter D, Mattson Mark P. Fasting: Molecular Mechanisms and Clinical Applications. Cell Metabolism. 2014;19(2):181-192. doi: https://doi.org/10.1016/j.cmet.2013.12.008
Collins N, Han SJ, Enamorado M, et al. The Bone Marrow Protects and Optimizes Immunological Memory during Dietary Restriction. Cell. 2019;178(5):1088-1101.e15. doi: https://doi.org/10.1016/j.cell.2019.07.049





